Business Plan
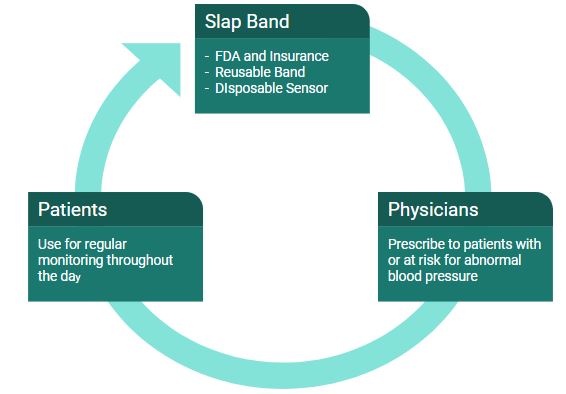
The Slap Band will first have to gain FDA approval as a class II medical device. This will be accomplished by gathering blood pressure data and proving the results are equivalent to current devices on the market under section 510(k) of the Food, Drug, and Cosmetic Act. To obtain reimbursement from insurance companies, we will demonstrate the superiority of our product to the current standard of blood pressure monitoring. This will be accomplished by following the guidelines set by the Center for Medicare and Medicaid Services. A proper code will also be obtained for new technology and outpatient use by publishing Class II clinical data. In order to optimize adoption rates of this device, physicians will be reminded every 5-7 days to prescribe this device to patients with high blood pressure.

When patients are prescribed the Slap Band, their data will be sent to a healthcare call center. If the device is not detecting any blood pressure from the user, an agent from this call center will contact the patient to ensure the user is alright. In the event the individual does not respond, the agent will then call 911.
Blood pressure data collected from the user can then be sold by the call center to the insurance companies for a physician to examine. If the physician notices any periods when their patient's blood pressure was high they can call the patient to get more information from them and potentially change the dosage of their blood pressure medication. This process removes liability from the physician. If physicians had direct access to continuous data they could leave themselves vulnerable to lawsuits for not responding fast enough to a patient's needs.

